Deeplex® Myc-TB
Advanced Diagnostic Test
for Tuberculosis Infections and Antibiotic Resistance
Powered by GenoScreen
Get a FREE tailored demonstration based on your needs.
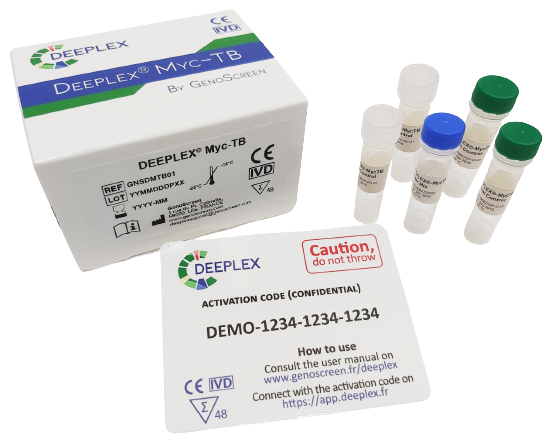
The all-in-one solution for extensive detection of Mycobacterium tuberculosis drug resistance
Deeplex Myc-TB is , developed by GenoScreen, is an NGS-based drug resistance testing solution for tuberculosis. In addition, it’s an innovative and integrated culture-free diagnostic kit, providing molecular results on resistance or susceptibility to 15 anti-tuberculosis drugs in less than 48 hours. The assay is based on targeted deep sequencing and automated data analysis via the secure Deeplex Myc-TB web application.
Furthermore, our product can detect tuberculosis heteroresistance down to 1-3%, and also identifies Mycobacterium tuberculosis complex (sub)lineages and spoligotypes, as well as more than 100 species of non-tuberculous mycobacteria (NTM).
By providing rapid and extensive prediction of genetic resistance to anti-tuberculosis drugs, this innovative test represents a powerful tool for guiding effective therapeutic management of patients suffering from tuberculosis. Thus, the NGS-based drug resistance testing for tuberculosis provided by Deeplex Myc-TB allows clinicians to detect both first- and second-line drug resistance.
15
anti-TB drugs
screened
48h
from clinical samples to results
1-3%
heteroresistance detection sensitivity
>100
mycobacterial
species identified
Key components of
Deeplex Myc-TB
- A Deeplex Master Mix: a single 24-plex PCR mix for culture-free amplification of 18 Mycobacterium tuberculosis drug resistance-associated gene targets combined with targets for mycobacterial species identification and MTBC strain genotyping.
- An internal control for in-sample quality control.
- A positive control for test validation.
- An activation code to access the Deeplex Myc-TB web application.
WHO consolidated guidelines on tuberculosis
Information sheet GenoScreen: Deeplex Myc-TB test
The World Health Organization (WHO) has recently updated its “Consolidated Guidelines on Tuberculosis“, recommending targeted next-generation sequencing (tNGS) as a preferred method for diagnosing drug-resistant tuberculosis (DR-TB). In particular this new guidance underscores the critical role of advanced diagnostics in quickly identifying drug resistance patterns, thereby helping clinicians make informed decisions and ultimately improving patient outcomes.
This NGS-based drug resistance testing for tuberculosis has been evaluated and endorsed by the WHO for its accuracy and efficiency.
Notably, Deeplex Myc-TB is highlighted by the WHO as the only tNGS assay meeting the performance criteria for all 10 drugs evaluated, making it the most effective solution for DR-TB diagnostics. Furthermore, by adopting Deeplex Myc-TB, healthcare providers gain access to the most advanced tNGS-based tool, thereby supporting faster and extensive diagnoses in alignment with WHO recommendations.
Download the technical note
Tuberculosis, a global public health challenge
Tuberculosis (TB) is amongst the most lethal infectious diseases globally, with 10 million new cases annually, resulting in nearly 1.5 million deaths each year.
Primarily, this contagious disease most often develops in the lungs, but can also affect multiple other parts of the body. It is caused by bacteria part of the Mycobacterium tuberculosis complex (MTBC). Treatment of active TB requires the uptake of combination of multiple antibiotics for 6 months or more.
Drug-resistant tuberculosis, a rising threat
The World Health Organization (WHO) estimated that about 450,000 new cases of multi-drug resistant (MDR) or rifampicin-resistant TB occurred in 2021, of which ~6% were extensively drug resistant tuberculosis (XDR-TB, additional resistance to at least one additional Group A drug).
When the MTBC strain that infects the patient is resistant to the drugs part of the treatment regimen, the therapy will be unsuccessful. Moreover, drug resistance can also amplify when pre-existing resistance is undetected. Thus, rapid, accurate and extensive detection of drug susceptibility and drug resistance is crucial for effective patient treatment, and to prevent further development of drug resistance and transmission of tuberculosis.
Our references
2023
Clinical utility of target-based next generation sequencing for drug-resistant TB
2022
Rapid molecular diagnostics
of tuberculosis resistance
by targeted stool sequencing
Sibandze D. B. et al.
Genome Medicine
2021
Deep amplicon sequencing for culture-free prediction of susceptibility or resistance to 13 anti-tuberculous drugs
Jouet A. et al.
European Respiratory Journal
2021
First molecular-based anti-TB
drug resistance survey in Eritrea
Mesfin A. B. et al.
The International Journal of Tuberculosis
and Lung Disease
2021
A comprehensive evaluation of GeneLEAD VIII DNA platform combined to Deeplex Myc-TB® assay to detect in 8 days drug resistance
to 13 antituberculous drugs and transmission
of Mycobacterium tuberculosis complex
directly from clinical samples
Bonnet I. et al.
Frontiers in Cellular and Infection Microbiology
2021
Targeted next generation sequencing directly from sputum for comprehensive genetic information on drug resistant Mycobacterium tuberculosis
Kambli P. et al.
Tuberculosis
2021
Rapid genomic first- and second-line drug resistance prediction from clinical Mycobacterium tuberculosis specimens using Deeplex® Myc-TB
Feuerriegel S. et al.
European Respiratory Journal
2020
Zoonotic tuberculosis in humans assessed by next-generation sequencing: an 18-month nationwide study
in Lebanon
El Achkar S. et al.
European Respiratory Journal
2020
A sister lineage of the Mycobacterium tuberculosis complex discovered
in the African Great Lakes region
Ngabonziza J. C. S. et al.
Nature Communication
2019
How well do routine molecular diagnostics detect rifampin heteroresistance
in Mycobacterium tuberculosis?
Kamela C.S. Ng et al.
Journal of Clinical Microbiology
2019
Drug-resistant tuberculosis,
Lebanon, 2016 – 2017
El Achkar S. et al.
Emerging Infectious Diseases
2018
Outbreak of multidrug-resistant tuberculosis in South Africa undetected by WHO-endorsed commercial tests:
an observational study
Makhado N. A. et al.
Lancet Infectious Diseases
Press releases
2025
GenoScreen and Clear Labs Announce the Launch of Clear Dx™ Deeplex® Myc-TB, an Automated Genomic Solution for Mycobacterium Characterization and TB Drug Resistance Detection
2022
Illumina and GenoScreen Partner to Expand Access to Genomic Testing for Multidrug-Resistant Tuberculosis


